All acids have a conjugate base. Acids donate H when they react.
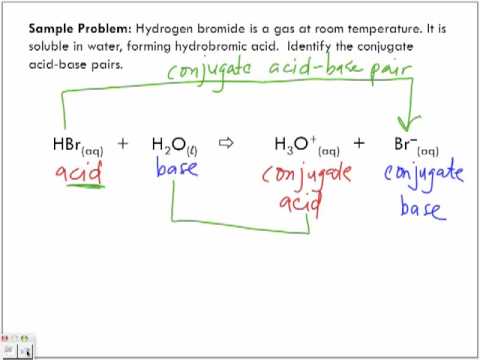
Conjugate Acid Base Pairs Sample Problems Youtube
Using the data in the table which of the conjugate bases below is the strongest base.

. You just studied 6 terms. Furthermore Which of the following is a conjugate acid base pair H2SO4 H 3 O is the conjugate acid of H 2 O and Cl is the conjugate base of HCl. A conjugate acid-base pair differs by one proton only.
What are conjugate acid-base pairs. 25 Questions Show answers. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H 2 O HSO 3 171 x 102 HSO 4 SO 4 2 120 x 102 H 3 PO 4 H 2 PO 4 752 x 103 FeH 2 O 6 3 FeH 2 O 5 OH 2 184 x 103 H 2 C 8 H.
It behaves as an acid. Find the conjugate acidbase for the following species. HCO3- H3O H2CO3 H2O c.
The conjugate acid-base for the given species is mentioned in the table below. D The equilibrium reaction will be In this reaction are act as a conjugate base-acid. The balanced reaction will be.
All of these are conjugate acid-base pairs. Well the simple answer is NO. Thus A HPO 32 PO 33 B H 2 PO 4 H PO 42.
Now H 3 PO 4 H H 2 PO 4 Here the acid H 3 PO 4 loses a proton to give H 2. In this reaction are act as a conjugate acid-base. The balanced reaction will be.
C The equilibrium reaction will be In this reaction are act as a conjugate acid-base. The weaker the conjugate base. Solution Verified by Toppr Correct option is D H 2 PO 4 PO 33 is not a conjugate acid-base pair as it differs by two protons.
Three doses of the diphtheriatetanusacellular pertussis DTaP vaccine at 2 4. Conjugate acid-base pair are compounds which differ by H Heres are two examples of conjugate acid-base pair. Identify the acid-conjugate base pair and the base-conjugate acid pair in the following chemical reaction HFaq NaOHaq H2Ol NaFaq The Nation is a spectator ion.
Three doses of the hepatitis B vaccine at birth and 1 and 6 months of age. Science Chemistry QA Library Identify the conjugate acid-base pairs in the following reactions. Although it has a negative charge it will never accept a H to form H2SO4 sulfuric acid Therefore the sulfate ion SO24 is the conjugate base.
Click again to see term. Tap card to see definition. The conjugates will always be listed on the product side of the reaction.
From the given options and represents a base-conjugate acid pair. All bases have a conjugate acid. On the other hand for a.
A mother brings her 18-month-old child to the clinic to receive the next scheduled vaccine. For example HCl and Cl ion differ by one proton only. HNO 2 CN HClO 4 F OH CO CO 3 2 - and S 2 Advertisement Remove all ads Solution A conjugate acid-base pair is a pair that differs only by one proton.
Among the following pairs the one representing a conjugate pair of an acid a base is. General-chemistry More questions like this Pneumococcal conjugate and pneumococcal polysaccharide vaccines can be given at the same time. H 3 O OH-H 2 SO 3 SO 3 2-NaOHNa SO 4 2-SO 3 2-.
H2PO4- H2O H3O HPO42- b. Which of the following is a conjugate acid-base pair. What is meant by the conjugate acid-base pair.
Finally Is H2SO4 and SO4 a conjugate acid-base pair Explanation. Tap again to see term. In this is a base which accepts a proton to form conjugate acid.
Conjugate acid-base pairs differ by which one of the following. In this is a base which accepts a proton to form conjugate acid. The child has previously received the following vaccines.
Now up your study game with Learn mode. NH 4 NH 3. What is the conjugate acid in the following equation.
What is the acid that reacts with this base when ammonia is dissolved in water. This is most easily seen when they dissociate in water. Group of answer choices.
The point is that there is some competition between the acid and its conjugate base for the hydronium ion such that the mathpHmath of the solution remains tolerably close to the mathpK_amath of the acid. According to the Bronsted Lowry conjugate acid-base pair concept an acid is a substance which donates protons and a base is a substance which accepts protons. OH H20 and HF F O HF F and OH H20 HF OH and H2O F O H20 F and HF OH.
According to the Bronsted Lowry conjugate acid-base pair concept an acid is a substance which donates protons and a base is a substance which accepts protons. Hence they are the conjugate acid-base pair. The concept of conjugate acid-base pair is related to Bronsted-Lowry acid-base theory and according to this theory acid is a proton H donor while base is a proton acceptor.
From the given options and represents a base-conjugate acid pair. H2O l NH4 aq NH3 aq H3O aq Click card to see definition. Medium Solution Verified by Toppr Correct option is A A conjugate acid-base pair is the one in which the acid and its conjugate base or vice versa differ in only one proton H From the given options there is only one such pair which is H 3 PO 4 and H 2 PO 4.
Ammonia acts as a weak base in aqueous solution. Asked Aug 9 2019 in Chemistry by Kate_Bona A. Some common examples of conjugate acid-base pairs are HClO 4 H ClO 4 H 2 SO 4 H HSO 4 HCl H Cl HNO 3 H NO 3 Check out the video given below to know more about the uses of acid and bases.
Which of the following is a conjugate acid-base pair. Among the following pairs the one representing a conjugate pair of an acid a base is. Lets focus on the first example CH_3COOH.
Using the data in the table which of the conjugate acids below is the strongest acid. Hence from this we conclude that the option d is not a conjugate acid-base pair but it is a act as conjugate base-acid. Buffers are formed from weaker acids OR weaker bases and their comparably weak conjugate pair.
HCO3- HSO3- CO32- H2SO3 Note. A conjugate acid is the species that. Among the following pairs the one representing a conjugate pair of an acid a base is.
Provide the whole solution. The stronger the acid. H 2SO4 H 2O H SO 4 H 3O In this example sulfuric acid H 2SO4 is an acid because it donates H to the water.
NH4 is an acid and NH3 is its conjugate base. Strong acid forms a weak conjugate base and vice versa.

Pin On Acids And Bases In Organic Chemistry

5 1 Acid Base Definitions Conjugate Acid Base Pairs General Chemistry For Gee Gees

How To Identify Conjugate Acid Base Pair Bronsted Lowery Arrhenius Youtube

0 Comments